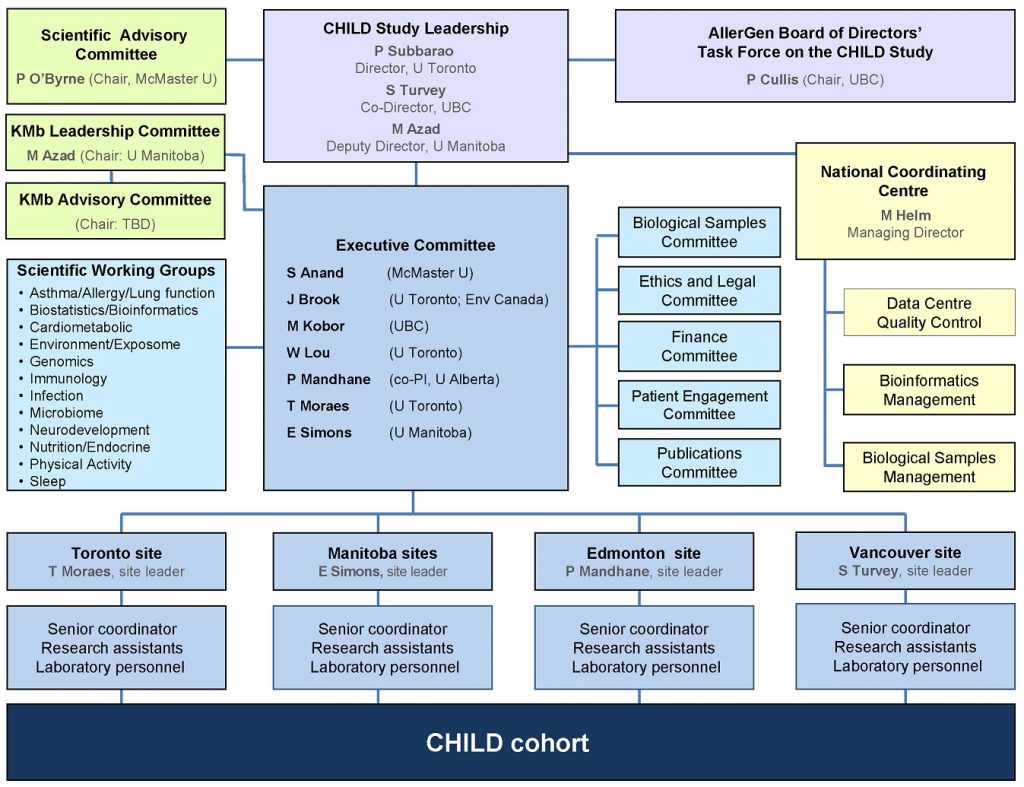
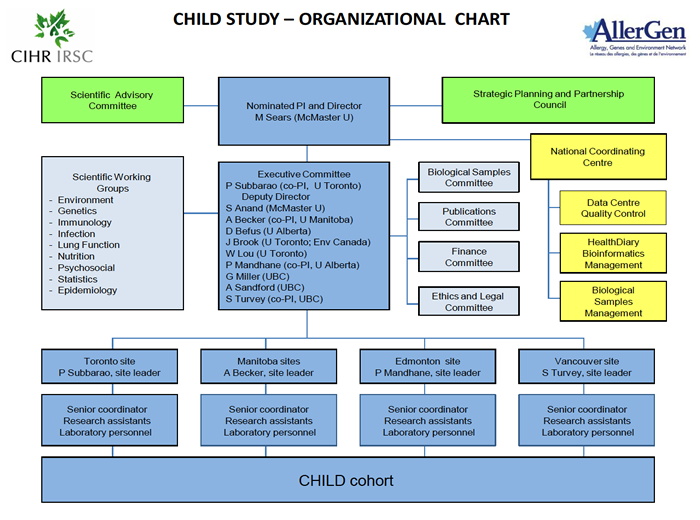
At the core of the CHILD Cohort Study’s governance and administration structure are a Director, Co-Director and an Executive Committee comprising the site leaders and research theme leaders.
The decisions of the Executive Committee are informed by input from a Scientific Advisory Committee, and its work complemented by that of various Scientific Working Groups and Sub-Committees.
On-the-ground implementation of the Study is undertaken by teams at each of the four Study sites, under the coordination of a National Coordinating Centre–within which Study data and biological samples are centralized and managed.
CHILD Cohort Study Organizational Chart
CHILD Cohort Study Directors

Director
Dr. Padmaja Subbarao
MD, MSc, FRCP(C)
Associate Chief, Clinical Research & Co-Lead, Precision Child Health, The Hospital for Sick Children
Staff Respirologist, Respiratory Medicine, The Hospital for Sick Children
Senior Scientist, Translational Medicine, Subbarao Lab
Professor, Department of Paediatrics and Dalla Lana School of Public Health, University of Toronto
Assistant Clinical Professor, Div. of Respirology, Dept. of Medicine, McMaster University
Canada Research Chair in Pediatric Asthma and Lung Health: Tier 1
Dr. Subbarao‘s primary research interest is in the early determinants and development of asthma. Specifically, she is studying changes in infant lung function and biomarkers of inflammation in infants, to elucidate the effect of environment and especially viruses on the development of early disease. Dr. Subbarao served as Deputy Director of the CHILD Cohort Study from 2007, became Co-Director in 2014, and then assumed the role of Director in July 2017.
Responsibilities: Among other things, the Director is responsible for the broad scope of conduct of the Study, including the maintenance of scientific and ethical standards, the well-being of CHILD participants, and the overall co-ordination of studies and personnel.

Co-Director
Dr. Stuart Turvey
MBBS, DPhil, FRCPC
Professor, Division of Allergy and Immunology, Department of Pediatrics, University of British Columbia
Aubrey J. Tingle Professor of Pediatric Immunology
Director, Clinical Research, and Investigator, BC Children’s Hospital
Dr.Turvey‘s research program is translational, interdisciplinary and unique in its focus on understanding the role of innate immunity in infectious and inflammatory diseases of childhood. Starting with a population of children with a defined infectious or inflammatory disease phenotype, he aims to determine the underlying cellular, molecular and genetic abnormalities responsible for the disease through detailed immunological, genomic and proteomic analysis. Dr. Turvey became Co-Director of the CHILD Cohort Study in July 2017.
Responsibilities: The Co-Director assists the Director in carrying out her responsibilities, including the formulation of policy and the day-to-day management of the Study.

Deputy Director
Dr. Meghan Azad
PhD
Associate Professor, Pediatrics & Child Health, and Canada Research Chair in Developmental Origins of Chronic Disease, University of Manitoba
Research Scientist, Children’s Hospital Research Institute of Manitoba
Population Health Pillar co-Lead, Manitoba Developmental Origins of Chronic Diseases in Children Network (DEVOTION)
Co-Director, Manitoba Interdisciplinary Lactation Centre (MILC)
Dr. Azad‘s research investigates how early-life exposures and experiences shape lifelong health. Her current research is focused on the role of maternal nutrition, infant feeding and human milk composition in growth and development during infancy and childhood. Dr. Azad became Deputy Director of the CHILD Cohort Study in February 2021.
Responsibilities: The Deputy Director leads/co-leads funding applications and projects to support core CHILD activities and increase the Study’s visibility; contributes to operational and strategic planning; and works with the Director and Co-Director to represent CHILD nationally and internationally among stakeholder groups.

Founding Director
Dr. Malcolm Sears
MB, ChB, FRACP, FRCPC, FAAAAI
Professor, Division of Respirology, Department of Medicine, McMaster University
AstraZeneca Endowed Chair in Respiratory Epidemiology
Researcher, St. Joseph’s Healthcare Hamilton
Allergy and Asthma Research Lead, Dunedin Multidisciplinary Health and Development Study
Chair, Publications Committee, CHILD Cohort Study
Dr. Sears has been involved in many studies investigating the epidemiology and natural history of asthma with particular focus on its frequency, risk factors and characteristics in large populations. He has been involved in the Dunedin Study for more than 30 years. Other of his research interests include assessment of asthma therapies, and the effects of indoor allergens, viral infections, air pollutants, smoking and hormones in respiratory disease.
Responsibilities: The Founding Director led the planning for the CHILD Cohort Study from initial discussions in 2004, was heavily involved in the development of the AllerGen Network for Centres for Excellence in 2005, and served as Director of the study from its inception in 2007 through to July 2017. He remains involved in the Study, especially in the reviewing and editing of publications, and maintains an advisory role as an ex officio member of the Executive Committee.
CHILD Cohort Study Site Leaders
The Study site leaders are co-PIs, and core members of the Executive Committee. They work with the Director and Deputy Director or Co-Director to implement the CHILD Cohort Study at each of the Study locations. Among other things, the site leaders are responsible for the conduct of the Study at their sites, including the maintenance of scientific standards, and fulfillment of ethical obligations.
Vancouver site leader

Dr. Stuart Turvey
MBBS, DPhil, FRCPC
Professor, Division of Allergy and Immunology, Department of Pediatrics, University of British Columbia
Aubrey J. Tingle Professor of Pediatric Immunology
Director, Clinical Research, and Investigator, BC Children’s Hospital
Edmonton site leader

Dr. Piush Mandhane
MD, PhD, FRCPC
Associate Professor, Department of Pediatrics, University of Alberta
Director, Division of Pediatric Respirology, Pulmonary & Asthma, University of Alberta
Pediatric Respirologist
Manitoba site leader

Dr. Elinor Simons
MD, PhD, MSc, FAAAAI, FAAP
Pediatric Allergist and Clinical Epidemiologist,, Children’s Hospital of Winnipeg
Assistant Professor, the University of Manitoba
Clinician-scientist, the Children’s Hospital Research Institute of Manitoba (CHRIM)
Toronto site leader

Dr. Theo Moraes
MD, PhD, FRCPC
Assistant Professor, Department of Paediatrics, University of Toronto
Scientist-Track Investigator, Translational Medicine, Sick Kids Research Institute
Staff Respirologist, Respiratory Medicine, The Hospital for Sick Children
National Coordinating Centre
The National Coordinating Centre (NCC) for the CHILD Cohort Study is the overall guardian of all data collected as part of the CHILD Cohort Study, regardless of the source of funding, including data collected through “add-on” studies. NCC staff also manage all the biological samples collected by the Study, maintain the online database containing all questionnaire responses, process all requests for data/biological samples/research resources, keep a record of media reports, track information requests and dissemination, support the frontline coordinators and researchers who work directly with families enrolled in the Study, coordinate ethics approvals, administer Study finances, and help with grant applications and report writing.
The NCC is hosted by McMaster University in Hamilton, Ontario. Contact information

Managing Director
Michelle Helm
PhD
Managing Director, CHILD Cohort Study

Program Manager
Elissa Brookes
Program Manager, CHILD Cohort Study

Research Associate
Bassel Dawod
MD, PhD, MSc
Research Associate, CHILD Cohort Study

Clinical Science Officer
Kozeta Miliku
MD, PhD
Clinical Science Officer, CHILD Cohort Study
Senior Research Fellow at McMaster University

Digital Initiatives Manager
Marshall Beck
Digital Initiatives Manager, CHILD Cohort Study

Senior Biostatistician
Vera Dai
MSc
Senior Biostatistician, CHILD Cohort Study; Biostatistician, Hospital for Sick Children

Statistical Analyst
Luis Ledesma
MSc
Statistical Analyst, CHILD Cohort Study

Research Coordinator
Kristina Szabo
MSc, PhD
Research Coordinator, CHILD Cohort Study

Administrative Coordinator
Dianne Tran
Administrative Coordinator, CHILD Cohort Study

Data Analyst
Xiao Xiao
Data Analyst, CHILD Cohort Study
The Executive Committee comprises the Study Co-Directors, the site leaders and research theme leaders. It meets regularly, at the call of the Director. Among other things, the Executive Committee is responsible for determining how the primary CHILD Cohort Study is conducted, who is involved and how it is funded. Decision making is a shared responsibility of the Executive.
Core members
Director, Co-Director, and site leaders
Adjunct members
Dr. Sonia Anand
Dr. Jeffrey R. Brook
Dr. Michael Kobor
Dr. Wendy Lou
The work of the Executive Committee is complemented by the supporting efforts of the following sub-committees.
Biological Samples Committee
The Biological Samples Committee (BSC) advises the Executive Committee on releasing CHILD samples for analysis. The BSC mandate is to ensure that proposed studies align with the goals and priorities of the CHILD Cohort Study and that samples are analysed efficiently by rigorous state-of-the-art methods, to maximise outcomes and minimise sample wastage.
Chair: Dr. Theo Moraes
Ethics and Legal Committee
Members: Prof. Timothy Caulfield, Dr. Nola Reis
Finance Committee
The Finance Committee is comprised of the Study Director (who Chairs the committee), Co-Director, Founding Director (ex-officio), site leaders, and Research Manager at the National Coordinating Centre. The Committee reviews the financial situation, cash flow, budgets, grant applications, and anticipated and extraordinary expenses of the Study, and determines allocation of funding across its components.
Knowledge Mobilization (KMb) Leadership Committee
The KMb Leadership Committee provides strategic direction and leadership to the CHILD Cohort Study’s knowledge mobilization activities. The Leadership Committee’s primary role is to identify, evaluate and prioritize knowledge mobilization, societal outreach, and commercialization opportunities relevant to CHILD Cohort Study findings.
Chair: Dr. Meghan Azad
Knowledge Mobilization (KMb) Stakeholder Advisory Committee
The KMb Stakeholder Advisory Committee is a multidisciplinary, cross-sectoral advisory committee that provides strategic implementation advice and feedback to the CHILD Cohort Study’s KMb Leadership Committee, toward mobilizing data and information arising from CHILD Cohort Study research results.
Chair: To be determined
Participant Engagement Committee
This committee ensures that the needs and experiences of parents and participants are included in the management and evolution of the CHILD Cohort Study, by bringing participants and Study staff together for broad discussion of important participant-related issues. These discussions help inform the development of Study visits, and the analysis and dissemination of Study information.
Chair: Dr. Meghan Azad
Publications Committee
The Publications Committee is comprised of the Director, Co-Director, two site leaders, two other Executive members and three elected investigators who serve a three-year term. The Publications Committee reviews and approves publication proposals submitted by investigators, reviews all abstracts and full papers prior to submission, and maintains a listing of all presentations of the CHILD Cohort Study.
The Scientific Advisory Committee (SAC) informs Study planning and decision-making by the Executive Committee.
Chair: Dr. Paul O’Byrne, Hamilton, Ontario, Canada
Dr. John Henderson, University of Bristol, UK
Dr. Fernando Martinez, Tucson Arizona, USA
Dr. Terrie Moffitt, Duke University, North Carolina, USA
Dr. Erika von Mutius, Munich, Germany
Dr. Peter Sly, Brisbane, Australia
Dr. Mark Raizenne, Ottawa
Dr. Felix Ratjen, Toronto, Ontario, Canada
To facilitate its many areas of specialized investigation, CHILD has developed various Working Groups consisting of researchers from related disciplines to develop the assessments, questionnaires and tools fundamental to meeting the objectives of the CHILD Cohort Study. These groups work in conjunction with the National Coordinating Centre to develop standard operating procedures for tests, biological sample collection and training for recommendation to, and approval by, the Executive Committee.
Scientific Working Groups (2017 onward)
Asthma, Allergy and Lung Function
This Working Group advises on the determination of asthma and wheezing phenotypes, the performance of lung function tests and of allergy skin tests. Lung function tests at age 8 years will include spirometry and methacholine challenge testing.
Chair: Dr. Padmaja Subbarao
Biostatistics and Bioinformatics
Data collection and management, and subsequent data analysis and integration are complex issues under continuing development and review by this Working Group.
Chair: Dr. Wendy Lou
Cardiometabolic Studies
This Working Group advises on the questionnaires, objective measurements and biological samples required to optimally assess cardiometabolic traits at age 8 years, including blood pressure, weight, height, waist circumference, body fat, step test for fitness, activity, screen time amount and type, outdoor play, diet, sleep patterns and duration.
Chair: Dr. Sonia Anand
Environmental and Exposome
The Environmental Working Group working with the National Coordinating Centre and Canada Mortgage and Housing Corporation developed Standard Operating Procedures for assessment of environmental exposures at each survey. Questions relate to the home, cleaning methods, heating and cooling, school, child and family activity, time spent in transit, mode of transport, use of swimming pools, and a multiplicity of chemical and environmental exposures. Outdoor air pollution is estimated by modeling.
Chair: Dr. Jeffrey R. Brook
Genomics
The Genomics Working Group advises on sample collection, storage and analysis of genetic material from parents and children, including epigenetics, and the broad field of ‘omics.
Chair: Dr. Michael Kobor
Immunology
The Immunology Working Group works with the National Coordinating Centre to develop Standard Operating Procedures for collecting, processing and quality control of blood samples. Blood components will be processed to provide RNA, DNA, whole blood, serum, plasma and peripheral blood mononucleocytes. Immunology investigations will focus on a comprehensive analysis of genetic, epigenetic, gene expression, innate and adaptive immunity and toll-like receptor profiles of children to understand the biological basis of allergic disease.
Co-Chairs: Dr. Thomas Eiwegger, Dr. Stuart Turvey
Infection
A primary focus of this Working Group is the detailed examination of respiratory infections in relation to outcomes of asthma and allergy.
Chair: Dr. Theo Moraes
Microbiome
This Working Group advises on sample collection and analyses for gut and respiratory microbiome studies.
Chair: Dr. Stuart Turvey
Neurodevelopment Studies
The Neurodevelopment Working Group advises on a broad range of assessments to follow the trajectories of mental illness, including anxiety and depression prodromes during development.
Chair: Dr. Peter Szatmari
Nutrition/Endocrine
The Nutrition Working Group works in conjunction with the National Coordinating Centre to develop assessment tools and implement food frequency questionnaires for the collection of nutritional information in mothers and children.
Co-Chairs: Dr. Meghan Azad, Dr. Russell de Souza
Physical Activity
The Physical Activity Working Group advises on questionnaires and measurement tools to assess the organized and unorganized physical activity of children in relation to disease outcome
Chair: Dr. Jeffrey Brook
Sleep
The Sleep Working Group works in conjunction with the National Coordinating Centre to develop assessment tools and implement questionnaire and objective assessment of sleep duration and disruption together with determination of media consumption.
Chair: Dr. Piush Mandhane