How to access CHILD Cohort Study data
Access policies for CHILD data and samples
There are 3 tiers of access to CHILD study data and biological samples:
1. Open access
Data types accessible in this tier include a list of study questionnaires, list of study samples collected, study protocols, and policies and procedures. These data are freely available on the CHILD website.
2. Registered access
Data types accessible in this tier include meta and aggregate data derived from questionnaires, other derived variables, test data, and biological sample data.
Temporary access is free for the purpose of developing a Concept Proposal to work with CHILD data and/or samples and is granted upon submission of a request to the NCC at child@mcmaster.ca.
The investigator requesting access must have an active academic or research appointment that allows the individual to:
- Pursue the proposed research;
- Engage in independent research activities; and
- Publish research results.
In his/her request, the investigator must provide the following information:
- Name
- Institution
- Institutional email and
- Phone number.
For CHILD Cohort Study (CHILD) microbiome data, researchers can access the publicly available raw sequencing reads deposited in various BioProjects. These datasets offer a flexible and rigorous starting point for high-quality microbiome research.
Integration of microbiota sequencing data with clinical endpoints, demographic data, environmental exposures, and other microbiota-associated profiles, such as stool metabolomics, is still supported through CHILDdb.
Raw microbiome datasets from CHILD currently available through BioProjects
| Data/Sample Type | Timepoint(s) | Sequencing Type | BioProject ID |
| Raw nasal microbiome data | 3M and 1Y | (16S rRNA gene sequencing) | PRJNA1127065 |
| Raw gut microbiome data | 3M and 1Y | (16S rRNA gene sequencing) | PRJNA657821 |
| Raw gut microbiome data | 3M and 1Y | (shotgun metagenomics) | PRJNA838575 |
| Raw milk microbiome data | 3M | (16S rRNA gene sequencing) | PRJNA481046 and PRJNA597997 |
- Technical Data: Minimal technical data (batch, exact age at sampling, visit number, and time from sample collection to long-term storage) are freely available upon request. Researchers may obtain this information by contacting the corresponding authors of the associated manuscripts or by emailing child@mcmaster.ca.
- Preprocessing Methods: Detailing preprocessing steps are available within published manuscripts citing these BioProjects. Depending on the age of the study, in some instances related code may also be available from the corresponding authors upon request.
- Data Linkage: Linkage of the samples to CHILD participant IDs and all other CHILD variables (including clinical endpoints, demographic data, and environmental exposures) will continue to require completion of the standard concept proposal process within CHILDdb.
3. Controlled access
Data types accessible in this tier include individual-level participant data derived from questionnaires, other derived variables, analysed biological sample data, test data, genomic/omic data, GPS and actigraphy data, linked datasets, and requested release of biological samples.
The investigator must submit a Concept Proposal via CHILDdb for approval. See the full process outlined below; see instructions for using CHILDdb here.
A cost-recovery fee will be applied.
The cost-recovery expenses for access to CHILD data and/or samples may include, but are not limited to:
- Administrative fee: $500 for a new Concept Proposal or $200 for a revision (amendment) to an approved Concept Proposal
- Data access fee: cost for questionnaire(s), derived and complex variables, calculated based on the size and complexity of the data request
- Sample retrieval fee: cost to retrieve biological samples from storage and prepare them for shipment to the investigator
- Sample shipment fee: cost to ship biological samples from storage to the Investigator’s analysis lab and from the Investigator back to storage (if applicable).
- Other fees: to be determined in the context of specific access requests as appropriate
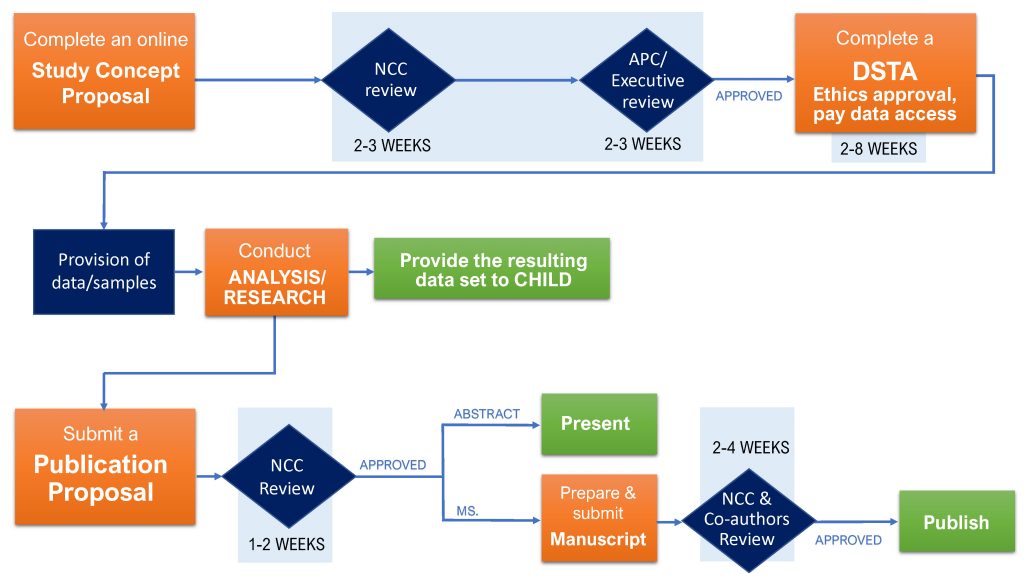
CHILD Cohort Study data/sample access process
1. Submit a Concept Proposal
To access CHILD Cohort Study data and/or biological samples for a collaborative project, and/or when applying for grant funding to pursue a project using CHILD data/samples, an investigator must submit a Concept Proposal (CP).
To complete a CP, the investigator must access CHILDdb by registering as a user and creating a new CP. (Step-by-step instructions in PDF and a tutorial video about using CHILDdb are available here.)
The completed Concept Proposal must include:
- Names of all students and investigators participating in the project;
- Details of project funding;
- Details of ethical review;
- Agreement to pay a data access fee and sample shipment fees, if applicable;
- Scientific outline (i.e., objectives, rationale, hypotheses and expected results, methods & analyses, sample size & statistical power);
- Study support details (i.e., expertise, resources, and infrastructure available);
- Justification as to why CHILD is the most appropriate source of data/samples for this proposal;
- Details of data being requested* (link variables);
- Details of biological samples being requested* (link samples), if applicable; and
- Disclosure of foreground and/or anticipated future Intellectual Property (IP) agreements or considerations.
* Ensure that your Concept Proposal anticipates and describes all the data and/or sample requests that you intend to make. If a data or sample request was not described in the approved Concept Proposal, the investigator must contact the NCC either to amend the existing proposal (which restarts the approval process) or to create a new one. In either case, data access will only be granted once the over-arching proposal has been approved.
Concept Proposal review:
Step one: NCC staff will perform an administrative review of each submission.
NCC staff will perform an administrative review of all submissions, against the following criteria:
- Availability of the requested data and/or the biological samples;
- “Depletability” of the requested biological samples, if applicable;
- Compatibility of the proposal with CHILD objectives;
- Eligibility of the investigator: If usage is for academic research purposes, the investigator must provide proof of his or her current appointment at a health institution and/or his or her status as an academic scientist is in good standing;
- Appropriateness of the non-academic (commercial) purpose of the research: If usage of data or biological samples is for commercial research purposes, such usage should be to increase knowledge in a health outcome project, which can lead ultimately to the development of a commercial product improving the health of Canadians;
- Compatibility of the proposal with the study participants’ consent;
- Potential duplication or overlap of the proposal with already approved Concept Proposals; and
- Ethics approval of the project (or proof that the investigator’s local REB does not require review for secondary use of data).
Step two: If the Study Concept Proposal meets all the criteria for the administrative review, the NCC will transfer it to the CHILD Access & Publications Committee (APC), and the CHILD Executive Committee (Executive), for a complete assessment.
If the Concept Proposal meets all the criteria of the administrative review, the CHILD Access & Publication Committee (APC) will then assess it against the following criteria:
- Scientific merit of the proposal (may involve review by a College of Reviewers);
- Feasibility of the proposal;
- Burden to the CHILD Cohort Study including, but not limited to, the costs and the use of resources;
- Suitability of the CHILD Cohort Study data for the proposal;
- Qualifications of the investigator;
- Number and credentials of research team members asking for access to data and/or biological samples;
- Rationale for research team member access;
- The investigator’s agreement to comply, via a Data and Sample Transfer Agreement (DSTA), with the CHILD Cohort Study policies (including, but not limited to, policies relating to the return of Actionable Research Results, the destruction of data and biological samples, confidentiality and security measures if part, or all, of the requested datasets are protected under first publication rights agreements); and
- Any overlap with IP considerations of other approved proposals.
If the data being requested is associated with a Data Consultant (e.g., omics data generated by another investigator, derived variables), the APC may recommend that the investigator collaborate with the Data Consultant for execution of the Concept Proposal.
The APC may also decide that the CHILD Executive Committee (Executive) should evaluate a Concept Proposal. In this case, the final approval or denial of the proposal is decided by the Executive. For all proposals involving a request for access to biological samples, a review by the Executive is mandatory.
Step three: Once the APC/Exec complete their reviews, a decision letter signed by the APC Chair and CHILD Director will be emailed to the investigator.
In the event of a positive decision, the investigator will also be sent a Data and Sample Transfer Agreement (DSTA), outlining the conditions of access, for signing.
2. Complete a DSTA, obtain ethics approval & pay data access fees
Once the Concept Proposal is approved, before any specific data or sample requests can be fulfilled, a Data and Sample Transfer Agreement (DSTA) must be established between McMaster University (the provider institution) and the requester’s institution, and signed by authorised representatives of both institutions as well as the CHILD Cohort Study Director and the recipient scientist.
Typically, DSTAs can take anywhere from 2–8 weeks for both institutions to execute.
The investigator must also obtain ethics approval for the proposed research and pay any applicable data and access sample fees.
Access to data/samples will only be allowed once the DSTA has been fully executed and the other requirements listed here are fulfilled.
3. Post-analysis: Provide the resulting dataset to CHILD
Data analyzed by investigators remains the property of the CHILD Cohort Study, and investigators are required to provide the NCC with documentation of derived data arising from analyses conducted using CHILD data and/or samples. It is not sufficient to provide the NCC with the final publication emerging from data analyses; a full dataset, including derived variables and coding scripts, must be provided.
4. Post-analysis: Submit a Publication Proposal and final manuscript
All research carried out following an approved Concept Proposal is expected to result in scholarly journal articles, monographs, books or presentations. The investigator must submit a Publication Proposal to CHILD for review for each anticipated publication deriving from the study described in the Concept Proposal, together with a completed Data User Responsibility Checklist. The investigator must also submit to CHILD for review any final manuscripts prior to their publication.
Abstracts, Posters, Presentations: An investigator who wishes to submit an abstract, poster or presentation to a national/international conference must submit his or her proposal via CHILDdb at least 2 weeks prior to the submission deadline. All abstracts must be linked to an approved Concept Proposal. Multiple Publication Proposals can be submitted for each Concept Proposal.
The expected timeline for approval is 1–2 weeks.
After an abstract is accepted for presentation as a poster or oral presentation, the investigator must upload the final version of the abstract, poster and/or presentation via CHILDdb. If the abstract is not accepted by the conference, the investigator must update his or her abstract request on CHILDdb.
Manuscripts
Proposal review: Prior to sending a manuscript to a journal for publication, the investigator must first submit a Publication Proposal via CHILDdb. Multiple Publication Proposals can be submitted for each Concept Proposal. Each publication proposal must be accompanied by a completed CHILD Cohort Study Data User Responsibility Checklist.
Once the proposal is submitted, the NCC/CHILD Access & Publication Committee (APC) will undertake a review of the proposal based to the following criteria:
- Compatibility of the Publication Proposal with the approved Concept Proposal; and
- The potential duplication or overlap with other approved Publication Proposals.
The NCC will notify the investigator once the proposal has been approved for submission.
Manuscript review: Once the investigator has drafted the publication and all co-authors have reviewed and approved submission of the manuscript, the APC will review the publication in accordance with, but not limited to, the following criteria:
- Adequacy of the description of the study sample and methods;
- Validity of basic statistical approaches;
- Scientific merit of the publication;
- Consistency of the publication with other CHILD Cohort Study publications;
- Quality of the publication (i.e., ensuring that the publication is of a high standard);
- Protection of CHILD Cohort Study participants’ confidentiality;
- Acknowledgment of the contributions of agencies or individuals that supported the CHILD Cohort Study and the CHILDdb. The following standard acknowledgement should be included as is or in a modified form to fit the journal’s requirements:
“We thank the CHILD Cohort Study (CHILD) participant families for their dedication and commitment to advancing health research. CHILD was initially funded by CIHR and AllerGen NCE Inc. Visit CHILD at childcohort.ca.”
Once the manuscript has been reviewed, the investigator will be sent a decision letter signed by the APC Chair. The expected timeline for review is 2–4 weeks. Approved manuscripts can then be submitted to journals for review.
If the manuscript is not accepted by a journal for publication and the investigator would like to modify the manuscript for submission to another journal, the investigator must notify the NCC.
Once the manuscript has been published, a PDF copy of the manuscript must be uploaded into Publication Proposal in CHILDdb.
Media protocol
The document below outlines a protocol for CHILD Cohort Study researchers to follow when dealing with the media to promote CHILD findings arising from a published research paper.
Guidelines for media interactions are essential to ensure that the CHILD Cohort Study, its research results, and its brand are professionally and consistently represented.
Close collaboration between the paper’s senior (corresponding) author, his or her institution and the AllerGen/CHILD Communications team is essential when planning and implementing public announcements of CHILD research findings.
CHILD Cohort Study Data User Responsibility Checklist
A completed CHILD Cohort Study Data User Responsibility Checklist, available below, must be included with your Publication Proposal submission.
CHILD Cohort Study Data User Responsibility Checklist (PDF)Summary of timelines (data access process)
The timeline between submission and approval/fulfillment of a proposal/request depends on the content and complexity of the proposal/request, as well as the existence of an active DSTA and REB review for the project.
- Concept Proposal for grant application purposes, requiring a Letter of Support = 2–3 weeks
- Concept Proposal requesting data only = 4–6 weeks
- Concept Proposal requesting data and samples (requires Exec review) = 4–8 weeks
- Abstract Requests = 1–2 weeks
- Manuscript Requests (CHILD Access & Publication Committee [APC], College of Reviewers) = 2–4 weeks